Innovative Medical Device Design: Enhancing Safety with Anti-Bacterial, PCT Sensing, and ESD Protection
In the rapidly evolving world of healthcare, the design of medical devices plays a critical role in ensuring patient safety and enhancing the effectiveness of clinical operations. With the increased demand for safer, more reliable medical devices, it is essential to integrate advanced technologies that address common issues such as bacterial contamination, touch sensitivity through gloves, and electrostatic discharge (ESD). This blog explores three key areas in medical device design: Anti-Bacterial Protection, PCT Sensing, and ESD Protection, highlighting how these innovations contribute to safer and more efficient healthcare environments.
Anti-Bacterial Protection in Medical Device Design
- Although bacteria will not live indefinitely on glass substrates, we are able to deploy Anti-Bacterial surface treatments using Nano Silver coatings, representing a long-term germ terminator which safely kills over 99.99% common disease-causing bacteria, fungi, and moulds.
- Integrating solely a cover lens or a full PCT Sensor infers an unavoidable air gap, which leaves Medical Devices susceptible to moisture and dust ingress.
- Our factory has a Class 10K Clean Room facility, with OCA (Optically Clear Adhesive), OCR (Optically Clear Resin) and OCF (Optically Clear Film) Bonding Machines, which enable the air gap to be filled with an index matched material.
- With Bonding material cured using a combination of UV and Autoclave processes, the resulting module is impervious to moisture or dust ingress and ultimately delivers a cleaner clinical HMI design solution.
- Clean sealing of the HMI to the client’s mechanical housing can be achieved using 3M VHB Gaskets, which will offer some degree of moisture protection, usually in the IP54 category, although IP65 sealing can also be provided depending on the customers requirement.
PCT Sensing for Medical Device Design
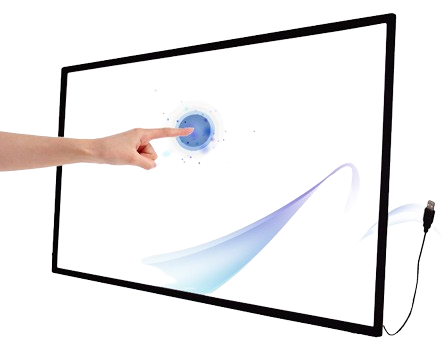
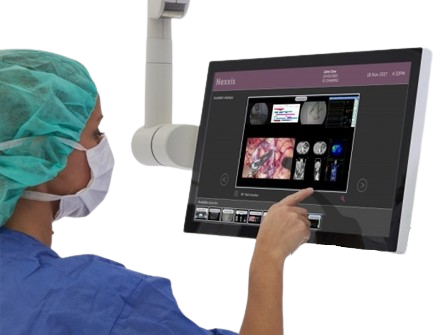
- The expectation in HMI Design is now for gloved touch operation, our partners have developed a range of PCT (Projected Capacitive Touch) Sensors capable of operation with Nitrile or even thicker gloves as standard.
- Clinicians need to be able to operate medical devices wearing surgical gloves, without concern for touch sensor drift or non-registered touches.
- The sensor design must incorporate an IC which can sense through both thicker cover lens materials and gloves, hence Startek calibrates PCT Sensor IC’s using Firmware to ensure that maximum activation occurs on the surface of the product within the client application.
- The chemically strengthened Soda Lime glass cover lens material is selected for maximum transmittance of light and enables upwards of 90% transmission from the display.
Click Below to Download our Brochure
ESD Protection in Medical Device Design
- Without sufficient monitoring and control, Electrostatic Discharge can pose a hazard to human safety within a clinical environment.
- Medical Devices are inherently vulnerable to static discharge and ESD build up from common clean room insulating materials like glass and polymers. ESD damage exhibits as unclassified or unknown device failures. Equipment may stop functioning without visible reason.
- Flammable gases and liquids utilized in a clinical setting are also a risk for ESD.
- ESD energy poses flammability risk, but also risk to accidental movement of patients and clinicians, leading to errors and potential accidents.
- We can mitigate risk with Projected Capacitive Cover Lens Design, integrating a fine mesh of ITO tracks into glass to offset Air Discharges of 15 kV, required for Level 4 of the IEC 61000-4-2 standard.
- ESD occurs when the human finger touches the front of an HMI, contacting for instance a Projected Capacitive Touch Sensor.
- If the current is allowed to run onto the touch sensor ITO traces, it can melt and fuse trace material, disabling sensing lines and causing various failure modes like phantom touches, or dead sections.
- Allowing for a sufficient border around the TFT Glass, by extending the Cover Lens, enables ESD to dissipate, through the chassis of the device, if the housing is correctly grounded.
- The Front Flush Mount HMI Design methodology illustrated on the right-hand side, offers the Design Engineer a compelling solution to all but the most intense ESD.
- We would not recommend a flush PCT Cover Glass Design, where the protective outer glass matches the viewable area of the display module. This would encourage ESD to run into the ITO traces of the underlying sensing layer, and cause damage.
- Whilst most modern Integrated Circuits have ESD protection design, for applications requiring high ESD immunity on some lines, additional external protection can be provided using inexpensive methods by employing single channel ESD protection or low pass filer coupled with ESD circuit si plastic package.
- There is also some safety net for Design Engineers by specifying a thicker cover lens design, although above 3mm, conventional PCT IC’s will require Firmware re-calibration to avoid limited sensing capability.
The design of medical devices is a complex process that requires careful consideration of various factors to ensure safety, reliability, and functionality. By incorporating advanced technologies such as Anti-Bacterial surface treatments, PCT Sensors, and ESD Protection, medical devices can better withstand the challenges of clinical environments, ultimately leading to improved patient outcomes and enhanced operational efficiency. As healthcare continues to evolve, staying at the forefront of these design innovations is essential for delivering the highest standard of care.